

blue
The photo above shows a recent dissolved oxygen test of my catfish pond. There are all kinds of water test kits out there and they have gotten more user friendly in the past decade. This kit uses small glass ampules that are filled with an indicator solution. When mixed with a water sample, the indicator will change color to "indicate" the amount of dissolved oxygen present. There is also a partial vaccuum in the ampule.
To test a sample, you fill a small vial with 25mL of the water to be tested. Then the ampule is inserted into the vial with the long skinny end of the ampule down in the vial. A little pressure breaks the sealed tip of the ampule allowing the right amount of your water sample to be pushed into the ampule due to the slight vaccuum in the ampule.
The ampule is then inverted a few times to mix things. Wait two minutes and then compare the color in the ampule to the color comparison set.
There's some judgement at this point depending on how color blind you are...but essentially the comparison tube closest to your sample color will tell you dissolved oxygen in ppm...parts per million.
My sample last Saturday at midmorning seemed to be between 6 and 8 ppm. The fish seemed happy and active with this level.
In a pond, oxygen levels will rise and fall with time of day. Typically, dissolved oxygen is highest during the peak photosynthesis hours and drops off at night when oxygen is being used, but photosynthesis has gone into idle. If you are going to have a low oxygen fish kill in your pond, it will probably happen in the morning since the fish were using it all night even though little oxygen was being released by plants in the dark.
The graph below (which I borrowed from the University of Florida/IFAS program) shows how oxygen levels change diurnally.
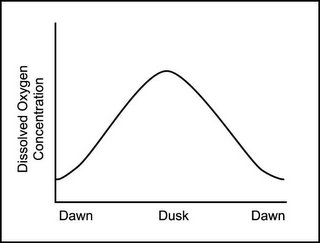
This applies to goldfish bowls with a fish and a plant, backyard goldfish ponds in Washington state or Pinellas county, empty (except for otters) fish ponds in Alabama, a frozen headwater lake in Michigan, aquaculture wannabe ponds in Florida,reservoirs in northern California, frozen ponds in Illinois or New York, and even potential ponds in Missouri.
One thing to remember is, plants do not make oxygen. In photosynthesis, the plant is trying to make glucose (sugar). To make a molecule of glucose, you need 6 atoms of Carbon, 12 atoms of Hydrogen, and 6 atoms of Oxygen. Photosynthesis breaks water and carbon dioxide apart to reassemble the parts (C,H,O) into glucose. After the glucose is formed there are some extra Oxygen atoms lying around and these are released as waste.
It's as if I gave you a little pirate ship made of 36 leggo blocks (that would be the water and carbon dioxide) and said tear it apart and make something new from those 36 blocks. You decide to make a cool tower (glucose molecule), but you only needed 24 blocks to make the tower perfect...the extra 12 blocks (oxygen) weren't needed. They are essentially waste as are the oxygen atoms given off by the plant in photosynthesis.
The more light, the more photosynthesis, the more waste (oxygen) the plants produce. It's easy to see why Oxygen levels in the pond climb during the day and drop at night.
Photosynthesis ...it's like a miracle, but it's not. It's a little like magic, but it's not.
It's just some fellow organisms passing gas.
Breathe deeply.
Breath deeply? I just exhaled because I was afraid there would be a test at the end of this chapter!! ;)
ReplyDeleteHowever, after reading this post, I think it's time to add more plants to our Goldfish Pond in Pinellas County - thanks for the science refresher course!
(Boy, you just come up with the most interesting things!!) LOL!
Not *totally* empty ponds in AL... we do still have a few grass carp... they must not be very tasty.
ReplyDeleteYou've made me want to hold my breath for a long, long time.
FC,
ReplyDeleteYeasts kind of perform the opposite reaction during fermentation. They feed on the sugars from the malted grains and the oxygen in the water. The waste that is produced is the alcohol(the buzz)and carbon dioxide (the bubbles) that we enjoy when sampling the brew.
I agree, it's sort of magical to see it actually taking place! Who knew that the freshman biology classes would ever pay off!
Now you've made me want to go test the levels in our pond. Great info here. I wish I had paid attention in my freshman biology class.
ReplyDeleteYou are a natural teacher...plus you make even the most mundane science stuff interesting.
ReplyDeleteQuestion, oh poohbah of Florida: Why test your water? Are you testing the levels so you can try to keep them at this level throughout the year or are you just interested? Or...did I miss something?
Maybe those kids who enjoy making the gas passing sounds are on to something. Thanks for the science lesson to you and to thunderdave whose opposite reaction is not quite as necessary but a lot more tastey.
ReplyDeleteLaura,
ReplyDeleteWas I too nerdy? Add those plants!
Rurality,
Grass carp eat your photosynthesizers ya know. I checked out otter live traps on the net....way expensive.
Thunder,
I have a brew question I'll email ya'...could be an educational post on T and L.
RD,
How could you not pay attention in biology? Math, or economics ...yawn, but bio? :)
Hick,
You guessed it. It's to maintain good levels of Oxygen. For instance if Oxygen was low in the pond, you would hold off on feeding the fish.
Mac,
If only the kids were passing oxygen instead of hydrogen sulfide!
That nicely clears everything up. Now all I need is a fish. And a bowl. And some gas passers.
ReplyDeleteHoss,
ReplyDeleteQwest might be your source for the latter.
That's a pretty series of blue tubes there. So that's how dissolved oxygen can be measured. Very clever.
ReplyDeleteIn addition to animal use of oxygen at night (and day) plants are also using it so that adds to the demand at night.
One reason for fish kills during red tides, and those also happen mostly at night - the alga in huge numbers uses up all the oxygen at night, which wasn't there to any great degree in the warm water in the first place.
Wayne,
ReplyDeleteThese ampule kits are available in other parameters also. Your coolwater, flowing creek is probably pretty high in the DO department.
Neat science stuff - learned more then all the books told me about photosynthesis. It makes sense. But why breath deeply - oh - the oxygen.
ReplyDeletedoubleknot,
ReplyDeleteHope it wasn't too much like school.